In today’s world of medical advancements, LTG clinical trials support has emerged as a cornerstone of innovative research and development. The landscape of healthcare is evolving at an unprecedented pace, and clinical trials play a pivotal role in bringing new treatments to patients. With LTG clinical trials support, researchers and institutions gain access to invaluable resources that enhance their ability to conduct thorough and effective studies. This article delves into various aspects of LTG clinical trials support, exploring its importance, processes, challenges, and benefits.
The Role of LTG Clinical Trials Support in Modern Research
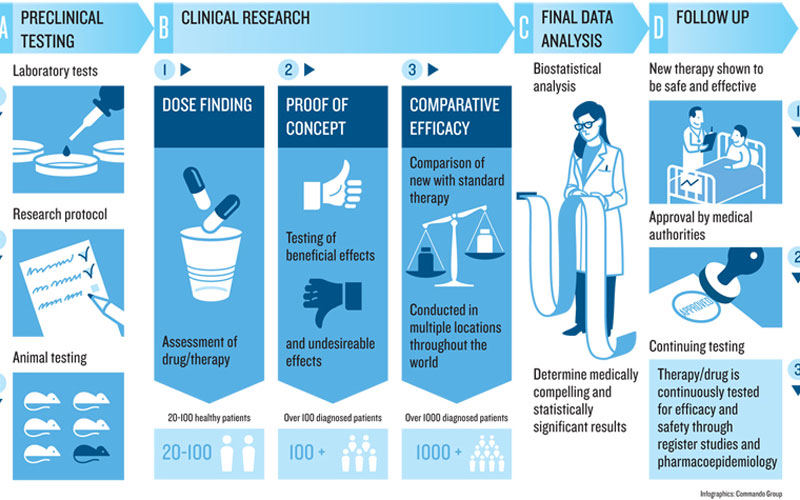
The significance of LTG clinical trials support cannot be overstated. Clinical trials are essential for testing new drugs, therapies, and techniques, ensuring they are both effective and safe for public use. LTG provides comprehensive assistance throughout this complex process, thereby enhancing the quality and efficiency of trials.
Understanding Clinical Trials
Clinical trials are systematic investigations designed to evaluate the effects of medical interventions on human subjects. They play a vital role in determining if new treatments can provide better outcomes than existing options.
Clinical trials typically consist of several phases, each with distinct objectives. These phases range from early-stage trials focused on safety assessment to later stages aimed at refining dosages and measuring efficacy compared to standard care. By understanding the structured approach of clinical trials, stakeholders can appreciate the complexities involved and the need for robust support systems like LTG.
The Importance of Support Systems
For clinical trials to succeed, they require extensive planning, coordination, and oversight. This is where LTG clinical trials support comes into play. Various aspects of trial management, such as site selection, patient recruitment, regulatory compliance, and data management, demand expert knowledge and resources.
Without adequate support, many clinical trials face significant hurdles, which may impede progress and lead to costly delays. Thus, having a dedicated partner like LTG ensures that trials are optimized for success by leveraging data-driven insights and best practices in trial design and execution.
Benefits of LTG Clinical Trials Support
Organizations that engage LTG clinical trials support enjoy numerous benefits including:
Enhanced efficiency in trial processes Improved patient recruitment and retention strategies Mitigation of regulatory risks Access to cutting-edge technology for data collection and analysis
With these advantages, clinical trials can operate more smoothly, ultimately leading to a faster path from research to real-world application.
Key Components of LTG Clinical Trials Support
The framework of LTG clinical trials support encompasses several essential components that work synergistically to facilitate successful trial outcomes.
Comprehensive Trial Design
A well-structured trial design is crucial for obtaining meaningful results. LTG applies scientific rigor and experience in developing protocols that align with regulatory requirements and scientific standards.
The design phase includes identifying primary and secondary endpoints, selecting appropriate control groups, and determining sample sizes. Each of these elements must be carefully considered to ensure the trial’s findings will have credibility and applicability in clinical practice.
Moreover, LTG’s expertise in adaptive trial designs allows for flexibility during the study, enabling modifications based on interim results. This adaptability can foster innovation in trial methodologies, propelling research forward in ways that traditional designs may not allow.
Patient-Centric Approaches
Recruiting diverse patient populations is critical for the generalizability of trial results. LTG clinical trials support emphasizes the importance of inclusive participant recruitment strategies that reflect real-world demographics.
By utilizing advanced analytics and community engagement, LTG helps identify potential participants who meet eligibility criteria while also addressing barriers to participation. This focus on inclusivity not only enhances the validity of trial findings but also promotes health equity.
Engaging patients early in the process further enriches the trial design. Feedback from potential participants can inform protocol adjustments, making the study more appealing and relevant to those it aims to serve.
Regulatory Compliance
Navigating regulatory landscapes can be daunting for organizations conducting clinical trials. LTG clinical trials support provides guidance on complying with various regulations, safeguarding against potential legal issues that could arise during the trial process.
This includes assistance in preparing and submitting necessary documentation to regulatory bodies, facilitating interactions, and ensuring adherence to ethical standards. By mitigating legal risks, LTG allows researchers to focus on generating high-quality data instead of worrying about compliance challenges.
Overcoming Challenges in Clinical Trials with LTG Clinical Trials Support
Despite the advantages of LTG clinical trials support, challenges persist in the realm of clinical research. Identifying these challenges and understanding how LTG aids in overcoming them is crucial for stakeholders seeking successful trial outcomes.
Recruitment and Retention Difficulties
One of the most significant challenges in clinical trials is recruiting and retaining participants. High dropout rates can compromise the integrity of trial results, leading to inconclusive findings.
LTG employs strategic recruitment tactics to overcome these obstacles, including outreach initiatives, partnerships with local healthcare providers, and leveraging digital platforms to connect with potential participants. By fostering relationships within communities, LTG enhances awareness and trust, encouraging participation in clinical research.
Retention strategies also play a key role. Providing clear communication, regular follow-ups, and incentives can significantly increase participant satisfaction and decrease dropout rates. LTG prioritizes these methods to ensure ongoing engagement throughout the trial’s duration.
Data Management and Integrity
Data management is another area fraught with challenges in clinical trials. Ensuring data accuracy and integrity is paramount for drawing valid conclusions from research findings.
LTG clinical trials support incorporates cutting-edge technology solutions for data collection and analysis. Electronic data capture systems enable real-time monitoring and minimize human error, providing reliable data sets for evaluation.
Additionally, LTG emphasizes rigorous data governance frameworks, establishing protocols for secure storage and management of sensitive information. This commitment to data integrity reinforces confidence in research outcomes, helping stakeholders make informed decisions based on trial results.
Technology Integration
As technology continues to reshape the healthcare landscape, integrating innovative tools into clinical trials has become increasingly vital. However, many organizations struggle with effectively incorporating these technologies.
LTG clinical trials support offers expertise in leveraging digital health solutions, such as telemedicine, wearables, and mobile applications. These technologies can enhance patient engagement, streamline data collection, and improve overall trial efficiency.
By embracing technology, clinical trials can reach broader populations, gather more nuanced data, and adapt to changing patient needs. Collaborating with LTG empowers organizations to harness the full potential of technological advancements in their research efforts.
FAQs
What are LTG clinical trials?
LTG clinical trials refer to a comprehensive support system provided by LTG, assisting organizations in planning, executing, and managing clinical trials efficiently. This includes areas like trial design, regulatory compliance, patient recruitment, and data management.
How does LTG help with patient recruitment?
LTG assists in patient recruitment by employing targeted outreach strategies, forming partnerships with healthcare providers, utilizing digital platforms, and prioritizing inclusivity to engage diverse populations. This multifaceted approach ensures a steady pool of eligible participants for clinical trials.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is essential in clinical trials to ensure the safety and rights of participants, maintain research integrity, and avoid legal repercussions. LTG provides expertise and guidance to navigate complex regulations and adhere to ethical standards throughout the trial process.
Can LTG support adaptive trial designs?
Yes, LTG specializes in adaptive trial designs, allowing for modifications based on interim results. This flexibility can enhance the relevance and impact of clinical trials, promoting innovations in treatment methodologies and ultimately benefiting patient care.
How can technology improve clinical trials?
Technology can improve clinical trials by enhancing patient engagement, streamlining data collection, and increasing trial efficiency. LTG integrates digital health solutions, such as telemedicine and mobile applications, to optimize trial processes and gather more comprehensive data.
Conclusion
In the ever-evolving field of medicine, LTG clinical trials support stands out as an indispensable ally for researchers and healthcare organizations alike. By providing expert guidance across various domains—including trial design, patient recruitment, regulatory compliance, and technology integration—LTG empowers stakeholders to navigate the complexities of clinical research effectively.
As we continue to explore innovative therapies and interventions, the role of LTG in supporting clinical trials will undoubtedly remain critical. Harnessing the power of collaboration, technology, and patient-centered approaches will shape the future of medical research, ultimately leading to improved outcomes and enhanced quality of life for patients worldwide.
