In the rapidly evolving landscape of pharmaceuticals, chemicals, and environmental safety, regulatory toxicology support LTG has emerged as a critical component in ensuring public health and safety. This niche area of toxicology encompasses a range of activities aimed at assessing the safety of substances and their potential risks to human health and the environment. As regulatory frameworks become more stringent and complex, the importance of robust toxicological evaluations cannot be overstated.
Understanding Regulatory Toxicology
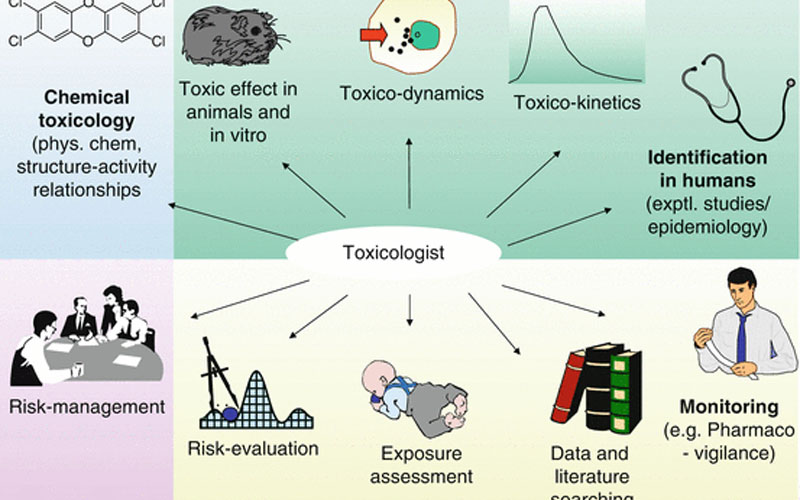
To appreciate the role of regulatory toxicology support, it’s essential to delve into its foundational aspects. This section will explore what regulatory toxicology entails, its significance in public health, and the interplay between science and regulation.
Defining Regulatory Toxicology
Regulatory toxicology is the branch of toxicology that focuses on the assessment and management of risks posed by chemical substances. It involves evaluating data gathered from laboratory studies, epidemiologic research, and other scientific inquiries to determine how substances may impact human health and the environment.
This field operates within a framework of regulations set forth by governmental agencies, ensuring that substances used in everyday products—from pharmaceuticals to cosmetics—meet established safety standards.
The goal of regulatory toxicology is to provide scientifically sound assessments that inform risk management decisions, ultimately protecting consumers and ecosystems alike.
The Role of Regulatory Agencies
Various regulatory agencies around the world—such as the U.S. Environmental Protection Agency (EPA), the European Medicines Agency (EMA), and the Food and Drug Administration (FDA)—play a pivotal role in overseeing the safety of substances. They depend heavily on regulatory toxicology support to evaluate the risk profiles of new and existing chemicals and pharmaceuticals.
These organizations set guidelines for conducting toxicological studies and interpreting the findings. By maintaining high standards, they ensure that only safe products enter the market, thereby safeguarding public health.
Importance in Public Health
The significance of regulatory toxicology extends beyond mere compliance with laws. It serves as a pillar for public health initiatives, shaping policies that protect communities from harmful exposures. Through rigorous assessments, regulatory toxicologists can identify potential hazards associated with chemical substances, contributing to informed decision-making processes that prioritize safety.
Moreover, effective regulatory toxicology support fosters transparency and builds trust between regulators, industry stakeholders, and the general public. By communicating risk assessments clearly, regulatory toxicologists help demystify scientific findings, enabling citizens to make informed choices about the products they use every day.
The Process of Toxicological Evaluation
Conducting a thorough toxicological evaluation is a multi-faceted process that requires expertise in various areas such as biochemistry, pharmacology, and environmental science. This section outlines the stages involved in this intricate process, emphasizing its complexity and importance.
Risk Assessment Framework
The cornerstone of regulatory toxicology lies in risk assessment—a systematic process that evaluates the likelihood of adverse effects resulting from exposure to a substance. This framework comprises four primary steps: hazard identification, dose-response assessment, exposure assessment, and risk characterization.
Hazard Identification This initial step involves determining whether a substance poses any potential health risks. Studies examining the substance’s toxicity are reviewed to identify any harmful effects.
Dose-Response Assessment Once hazards are identified, scientists analyze the relationship between the dose and the severity of the effect. This helps establish safe exposure levels and informs recommendations for product use.
Exposure Assessment
Understanding how people and the environment interact with substances is crucial. Exposure assessment evaluates the extent, frequency, and duration of exposure to a chemical or drug. Factors like age, sex, and underlying health conditions are considered to personalize risk assessments.
By comprehensively analyzing exposure scenarios, toxicologists can present clearer insights into the risk magnitude associated with a substance. This allows for informed regulatory decisions that balance societal benefits with safety concerns.
Risk Characterization
The final step in the risk assessment process synthesizes the information gathered from previous steps. In this phase, toxicologists communicate their findings regarding the level of risk associated with the substance, often including recommendations for risk management strategies.
Risk characterization is vital for supporting regulatory actions. It ensures that stakeholders understand both the potential hazards and the safety measures to mitigate risks, fostering an environment of accountability and transparency.
Challenges in Regulatory Toxicology
As the field of regulatory toxicology evolves, it faces numerous challenges that can impede effective safety assessments. This section discusses some of these obstacles and how the industry can adapt to overcome them.
Evolving Regulations
Regulatory frameworks are continually being updated to keep pace with new scientific findings and emerging technologies. However, these changes can create confusion and uncertainty among manufacturers and researchers.
Staying compliant with shifting regulations necessitates continuous education and training for professionals in the field. Ongoing collaboration among scientists, regulatory agencies, and industry stakeholders is essential to navigate the complexities of evolving regulations effectively.
Data Gaps and Limitations
Another significant challenge in regulatory toxicology support is the availability and quality of data. In many cases, chemicals may lack sufficient toxicological data, making it difficult to conduct comprehensive evaluations. Additionally, reliance on animal testing raises ethical concerns while limiting the applicability of results to humans.
Advancements in alternative testing methods, such as in vitro testing and computational models, hold promise for addressing these limitations. Greater investments in innovative research can lead to the generation of more relevant data that supports safety assessments.
Balancing Innovation and Safety
As industries innovate and introduce new products, the need for timely evaluations becomes increasingly pressing. However, the desire for speed must not compromise safety. Striking a balance between fostering innovation and ensuring public health is paramount.
Regulatory toxicology must proactively collaborate with innovators to streamline assessment processes without sacrificing thoroughness. Developing clear communication channels can help expedite reviews while maintaining safety standards.
Future Directions in Regulatory Toxicology
The future of regulatory toxicology holds exciting possibilities shaped by technological advancements and increased interdisciplinary collaboration. This section explores emerging trends likely to influence the field.
Integrative Approaches
The integration of various scientific disciplines into regulatory toxicology is gaining traction. By combining insights from chemistry, biology, and data science, toxicologists can better predict how substances behave in complex biological systems.
Such integrative approaches allow for more accurate assessments, enhancing the precision of risk evaluations. This shift toward collaboration emphasizes the interconnected nature of science and reinforces the need for holistic methodologies.
Advances in Technology
Technological innovations, particularly artificial intelligence (AI) and machine learning, are revolutionizing toxicological assessments. These tools facilitate data analysis at unprecedented speeds, enabling toxicologists to process large datasets and predict outcomes more effectively.
Automation also reduces human error, streamlining the evaluation process. As technology continues to evolve, regulatory toxicology will benefit significantly, enhancing the efficiency and accuracy of safety assessments.
Emphasizing Transparency and Communication
In an era of increasing public scrutiny, transparency in regulatory toxicology will become even more critical. Stakeholders demand clear communication about risks and benefits associated with products, emphasizing the importance of transparent processes in building trust.
Regulatory agencies and toxicologists must adopt strategies to engage with the public effectively. Providing accessible information and creating platforms for dialogue can empower individuals and communities to participate in discussions regarding safety and regulation.
FAQs
What is regulatory toxicology support?
Regulatory toxicology support refers to the expertise and assistance provided in assessing the safety of chemicals and pharmaceuticals. It involves conducting evaluations and communicating risk assessments to facilitate compliance with regulatory standards.
Why is regulatory toxicology important?
It plays a crucial role in ensuring public health and safety by evaluating potential risks associated with chemical substances. Effective regulatory toxicology support informs regulatory decisions that protect consumers and the environment.
What challenges does regulatory toxicology face?
Some challenges include evolving regulations, data gaps, limitations in traditional testing methods, and balancing innovation with safety. Addressing these challenges requires collaboration among stakeholders and investment in new methodologies.
How do advances in technology impact regulatory toxicology?
Technological advancements, such as AI and machine learning, enhance data analysis, streamline evaluation processes, and improve risk predictions. These innovations are transforming regulatory toxicology by increasing efficiency and accuracy.
What is the future direction of regulatory toxicology?
Future directions include integrative approaches combining various scientific disciplines, continued advancement in technology, and a focus on transparency and communication. These trends will shape the field and enhance safety assessments moving forward.
Conclusion
Regulatory toxicology support is an indispensable aspect of public health and safety, navigating the complexities of chemical evaluations and risk assessments. As the field continues to evolve, embracing innovative methodologies and interdisciplinary collaborations will be essential. By addressing challenges and prioritizing transparency, regulatory toxicology can foster a safer environment while promoting consumer confidence. With ongoing advancements paving the way for better practices, the future of regulatory toxicology looks promising indeed.
